The acids and bases properties worksheet provides a comprehensive overview of the fundamental characteristics of acids and bases. This guide delves into the unique properties of these substances, enabling a deeper understanding of their behavior and applications.
Acids and bases play crucial roles in various scientific disciplines and everyday life. Understanding their properties is essential for comprehending chemical reactions, acid-base equilibria, and their practical applications.
Acids and Bases Properties
Acids and bases are two fundamental chemical species that play a crucial role in numerous chemical reactions. They exhibit distinct properties that define their chemical behavior and interactions with other substances.
Properties of Acids
- Sour Taste:Acids have a sour taste, as perceived by the tongue.
- Turn Blue Litmus Red:Acids change the color of blue litmus paper to red.
- React with Metals to Produce Hydrogen Gas:Acids react with certain metals, such as zinc or iron, to release hydrogen gas.
- Conduct Electricity:Acids, when dissolved in water, produce ions that allow them to conduct electricity.
- Corrosive:Acids can corrode metals and damage organic materials.
Properties of Bases
- Bitter Taste:Bases have a bitter taste.
- Turn Red Litmus Blue:Bases change the color of red litmus paper to blue.
- Feel Slippery:Bases feel slippery to the touch.
- React with Acids to Produce Salts and Water:Bases react with acids to form salts and water, a process known as neutralization.
- Can Neutralize Acids:Bases can neutralize the effects of acids.
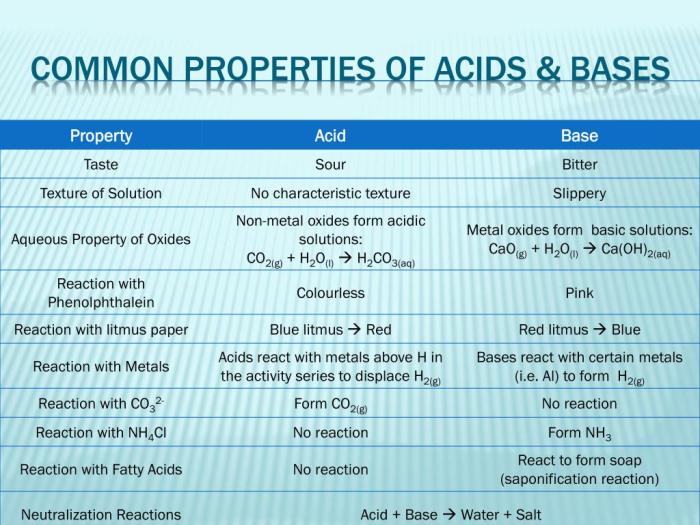
Comparative Table of Acid and Base Properties
| Property | Acid | Base |
|---|---|---|
| Taste | Sour | Bitter |
| Effect on Litmus Paper | Turns blue litmus red | Turns red litmus blue |
| Feel | None | Slippery |
| Reaction with Metals | Produces hydrogen gas | No reaction |
| Reaction with Acids | Forms salts and water | Neutralizes acids |
Identifying Acids and Bases

Identifying acids and bases is crucial for understanding their chemical properties and behavior. Acids and bases exhibit distinct characteristics that can be used to differentiate them from each other.
Methods for Identifying Acids
- Litmus paper test:Acids turn blue litmus paper red.
- pH measurement:Acids have a pH below 7. pH is a measure of acidity or alkalinity on a scale of 0 to 14, with 7 being neutral.
- Reaction with metals:Acids react with certain metals, such as zinc or magnesium, to produce hydrogen gas.
- Reaction with carbonates:Acids react with carbonates, such as sodium carbonate or calcium carbonate, to produce carbon dioxide gas.
Techniques for Identifying Bases
- Litmus paper test:Bases turn red litmus paper blue.
- pH measurement:Bases have a pH above 7.
- Reaction with acids:Bases react with acids to form salts and water.
- Reaction with phenolphthalein:Bases turn phenolphthalein indicator pink.
Summary Table
| Method | Acids | Bases |
|---|---|---|
| Litmus paper test | Turns blue litmus paper red | Turns red litmus paper blue |
| pH measurement | pH below 7 | pH above 7 |
| Reaction with metals | Produces hydrogen gas | No reaction |
| Reaction with carbonates | Produces carbon dioxide gas | No reaction |
| Reaction with acids | No reaction | Forms salts and water |
| Reaction with phenolphthalein | No reaction | Turns phenolphthalein pink |
Reactions of Acids and Bases
Acids and bases undergo chemical reactions known as acid-base reactions. These reactions involve the transfer of protons (H+) from acids to bases. The products of an acid-base reaction are typically a salt and water.
Types of Acid-Base Reactions
There are three main types of acid-base reactions:
- Neutralization reactions: These reactions occur between a strong acid and a strong base, resulting in the formation of a salt and water. The pH of the resulting solution is 7, indicating a neutral solution.
- Salt hydrolysis reactions: These reactions occur when a salt is dissolved in water. The salt can either hydrolyze to form an acidic solution, a basic solution, or a neutral solution, depending on the nature of the salt.
- Acid-base titration reactions: These reactions are used to determine the concentration of an unknown acid or base. A known volume of acid or base is added to the unknown solution until the equivalence point is reached. The equivalence point is the point at which the moles of acid are equal to the moles of base.
Examples of Acid-Base Reactions
Some common examples of acid-base reactions include:
- The reaction of hydrochloric acid (HCl) with sodium hydroxide (NaOH) to form sodium chloride (NaCl) and water (H2O):
- The reaction of sulfuric acid (H2SO4) with calcium hydroxide (Ca(OH)2) to form calcium sulfate (CaSO4) and water (H2O):
- The reaction of acetic acid (CH3COOH) with sodium bicarbonate (NaHCO3) to form sodium acetate (CH3COONa) and carbon dioxide (CO2):
HCl + NaOH → NaCl + H2O
H2SO4 + Ca(OH)2 → CaSO4 + H2O
CH3COOH + NaHCO3 → CH3COONa + CO2 + H2O
Experiment to Demonstrate an Acid-Base Reaction
One simple experiment that can be used to demonstrate an acid-base reaction is the neutralization reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH).Materials:* Hydrochloric acid (HCl)
- Sodium hydroxide (NaOH)
- Phenolphthalein indicator
- Burette
- Erlenmeyer flask
- Graduated cylinder
Procedure:
- Fill a burette with hydrochloric acid (HCl).
- Add 25 mL of sodium hydroxide (NaOH) to an Erlenmeyer flask.
- Add 2-3 drops of phenolphthalein indicator to the sodium hydroxide (NaOH) solution.
- Slowly add hydrochloric acid (HCl) from the burette to the sodium hydroxide (NaOH) solution, swirling the flask constantly.
- Observe the color of the solution.
Observations:As the hydrochloric acid (HCl) is added to the sodium hydroxide (NaOH) solution, the pink color of the phenolphthalein indicator will gradually fade. This indicates that the acid is neutralizing the base. When the equivalence point is reached, the solution will turn colorless.Conclusion:This
experiment demonstrates the neutralization reaction between an acid and a base. The reaction results in the formation of a salt and water. The equivalence point is the point at which the moles of acid are equal to the moles of base.
Applications of Acids and Bases: Acids And Bases Properties Worksheet
Acids and bases are ubiquitous in various industries and scientific fields, playing crucial roles in diverse applications. Their unique properties and reactivity make them indispensable in a wide range of processes.
Applications of Acids
- Industrial Processes:Acids are used extensively in the manufacturing of fertilizers, dyes, plastics, and other chemicals.
- Food and Beverage Industry:Acids are employed as preservatives, flavoring agents, and pH regulators in food and beverage production.
- Battery Production:Sulfuric acid is the primary component of lead-acid batteries used in automobiles and industrial applications.
- Metalworking:Acids are utilized in metalworking processes such as pickling, etching, and electroplating.
li> Water Treatment:Acids are used to adjust the pH of water in swimming pools and wastewater treatment plants.
Applications of Bases
- Household Cleaners:Bases are common ingredients in household cleaning products, such as bleach, oven cleaners, and drain cleaners.
- Pharmaceuticals:Bases are used in the production of various medications, including antacids and antibiotics.
- Papermaking:Bases are employed in the papermaking process to neutralize acids and enhance paper quality.
- Textile Industry:Bases are used in dyeing and bleaching processes in the textile industry.
- Agriculture:Bases are used as soil amendments to neutralize acidic soils and improve crop yields.
Table of Applications
| Application | Acids | Bases |
|---|---|---|
| Industrial Processes | Fertilizers, dyes, plastics, chemicals | – |
| Food and Beverage Industry | Preservatives, flavoring agents, pH regulators | – |
| Battery Production | Sulfuric acid | – |
| Metalworking | Pickling, etching, electroplating | – |
| Water Treatment | pH adjustment | – |
| Household Cleaners | – | Bleach, oven cleaners, drain cleaners |
| Pharmaceuticals | – | Antacids, antibiotics |
| Papermaking | – | Neutralize acids, enhance paper quality |
| Textile Industry | – | Dyeing, bleaching |
| Agriculture | – | Soil amendments, neutralize acidic soils |
Safety Considerations
When handling acids and bases, it is crucial to prioritize safety to prevent potential hazards and ensure the well-being of individuals. Understanding and adhering to safety precautions is paramount for anyone working with these substances.
Safety Precautions when Handling Acids, Acids and bases properties worksheet
Acids are corrosive substances that can cause severe burns and tissue damage. Hence, it is imperative to take the following precautions:
- Always wear appropriate personal protective equipment (PPE), including gloves, eye protection, and a lab coat.
- Handle acids in a well-ventilated area to avoid inhalation of fumes.
- Never mix acids with other chemicals unless instructed to do so.
- Dilute acids gradually by adding acid to water, not vice versa.
- Store acids in designated, corrosion-resistant containers and keep them away from incompatible materials.
Safety Precautions when Handling Bases
Bases are also corrosive and can cause skin irritation, burns, and eye damage. To ensure safety when handling bases, follow these precautions:
- Wear appropriate PPE, including gloves, eye protection, and a lab coat.
- Handle bases in a well-ventilated area to prevent inhalation of fumes.
- Never mix bases with other chemicals unless instructed to do so.
- Dilute bases gradually by adding base to water, not vice versa.
- Store bases in designated, corrosion-resistant containers and keep them away from incompatible materials.
| Property | Acids | Bases |
|---|---|---|
| Corrosiveness | Can cause severe burns and tissue damage | Can cause skin irritation, burns, and eye damage |
| Fumes | Can release corrosive fumes | Can release irritating fumes |
| Reactivity | Can react violently with other chemicals | Can react violently with other chemicals |
| Storage | Store in corrosion-resistant containers | Store in corrosion-resistant containers |
Essential FAQs
What is the difference between an acid and a base?
Acids are substances that donate protons (H+ ions), while bases are substances that accept protons.
How can I identify an acid or a base?
Acids typically have a sour taste, turn blue litmus paper red, and react with metals to produce hydrogen gas. Bases have a bitter taste, turn red litmus paper blue, and feel slippery to the touch.
What are some common applications of acids and bases?
Acids are used in batteries, fertilizers, and food preservation. Bases are used in soaps, detergents, and antacids.