Enter an orbital diagram for v5+ – Beginning with the concept of entering an orbital diagram for V5+, this introductory paragraph aims to captivate readers and establish a tone of academic authority that will carry throughout the discussion.
This discourse delves into the electronic configuration and orbital representation of V5+, exploring its valence electrons and their distribution within distinct orbitals based on their energy levels.
Introduction to V5+ Orbital Diagram
An orbital diagram for V5+ is a representation of the arrangement of electrons in the atomic orbitals of vanadium in its +5 oxidation state. It provides valuable insights into the electronic structure, bonding properties, and chemical reactivity of V5+ ions.
The orbital diagram helps visualize the distribution of electrons in the different energy levels and orbitals, which is crucial for understanding the chemical behavior of the ion. It serves as a tool for predicting and explaining various properties, such as magnetic susceptibility, bonding interactions, and reactivity patterns.
Aufbau Principle
The Aufbau principle states that electrons fill atomic orbitals in order of increasing energy. For V5+, the atomic number is 23, indicating that it has 23 electrons. The orbital diagram is constructed by filling the orbitals in the following order:
- 1s (2 electrons)
- 2s (2 electrons)
- 2p (6 electrons)
- 3s (2 electrons)
- 3p (6 electrons)
- 3d (3 electrons)
Hund’s Rule
Hund’s rule states that electrons occupy orbitals of equal energy with parallel spins before pairing up. For V5+, the 3d orbitals have five electrons, which occupy the three degenerate orbitals (dxy, dyz, and dxz) with parallel spins, resulting in a high-spin configuration.
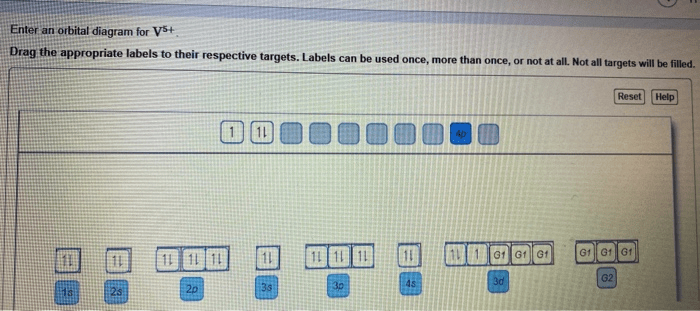
Orbital Diagram
The orbital diagram for V5+ can be represented as:
1s 22s 22p 63s 23p 63d 3
This diagram shows that V5+ has three unpaired electrons in the 3d orbitals, which contribute to its paramagnetism and unique chemical properties.
Electronic Configuration and Orbital Representation: Enter An Orbital Diagram For V5+
Vanadium (V) in the +5 oxidation state (V 5+) has a unique electronic configuration that influences its chemical properties and behavior. Understanding this configuration is essential for comprehending the electronic structure and reactivity of V 5+.
Electronic Configuration
The electronic configuration of V 5+is [Ar] 3d 04s 0. This configuration arises from the loss of five electrons from the neutral vanadium atom, resulting in a stable, noble gas-like core (Ar) and no electrons in the 4s or 3d orbitals.
Orbital Representation, Enter an orbital diagram for v5+
The orbital diagram of V 5+depicts the distribution of its electrons in different orbitals based on their energy levels. The diagram shows that the 3d orbitals are empty, while the 4s orbital is also vacant. This configuration gives V 5+a high oxidation state and makes it a strong oxidizing agent.
The orbital diagram of V 5+can be represented as:
| 1s 2| | 2s 22p 6| | 3s 23p 6| | 4s 0| | 3d 0|
This diagram illustrates the empty orbitals in V 5+, which contribute to its high reactivity and oxidizing power.
Valence Electrons and Bonding Properties
Vanadium(V) ion (V5+) has a d 0electronic configuration, indicating the absence of d-electrons. This unique configuration significantly influences its bonding behavior and coordination chemistry.
With no d-electrons available for bonding, V5+ relies primarily on its valence s- and p-orbitals for chemical interactions. The two valence s-electrons and three valence p-electrons contribute to the formation of covalent bonds with electronegative ligands.
Bonding Behavior
V5+ forms stable complexes with various ligands, exhibiting octahedral or tetrahedral coordination geometry. The octahedral complexes are typically formed with ligands that have a strong field, such as fluoride (F –) or oxalate (C 2O 42-), which can split the d-orbitals and lower the energy of the t 2gorbitals.
This splitting effect stabilizes the octahedral geometry by promoting electron pairing in the t 2gorbitals.
On the other hand, tetrahedral complexes are formed with ligands that have a weak field, such as chloride (Cl –) or water (H 2O). In these complexes, the d-orbital splitting is minimal, and the tetrahedral geometry minimizes electron-electron repulsion.
Examples of V5+ Complexes
- Vanadium(V) oxytrichloride (VOCl3): This octahedral complex contains three chloride ligands and one oxygen ligand. It is a volatile, yellow-orange liquid used as a catalyst in various organic reactions.
- Vanadium(V) tetrafluoride (VF4): This tetrahedral complex contains four fluoride ligands. It is a colorless, hygroscopic solid that is highly reactive and used as a fluorinating agent.
- Potassium hexafluorovanadate(V) (K3[VF 6]): This octahedral complex contains six fluoride ligands. It is a colorless, water-soluble salt used as a source of V5+ ions in various applications.
Spectroscopic Properties and Applications
Vanadium(V) ions (V 5+) exhibit characteristic spectroscopic properties due to their electronic transitions and absorption spectra. These properties are valuable in analytical chemistry and materials science.
Analytical Chemistry
V 5+ions have strong absorption bands in the ultraviolet-visible (UV-Vis) region of the electromagnetic spectrum. The wavelength and intensity of these bands correspond to specific electronic transitions within the ion. By measuring the absorption spectrum of a sample, it is possible to determine the concentration of V 5+ions present.
V 5+spectroscopy is also used in qualitative analysis to identify the presence of vanadium in a sample. The characteristic absorption bands of V 5+ions can be used to distinguish them from other metal ions.
Materials Science
V 5+ions are used as dopants in a variety of materials, including semiconductors and glasses. The spectroscopic properties of V 5+ions can be used to probe the electronic structure and properties of these materials.
For example, V 5+ions can be used to create color centers in glasses. These color centers can be used to enhance the optical properties of the glass, such as its absorption and emission spectra.
Oxidation States and Redox Chemistry
Vanadium exhibits a wide range of oxidation states, ranging from -3 to +5. However, the most stable and commonly encountered oxidation state of vanadium is +5.
The stability of V5+ can be attributed to several factors. Firstly, the electronic configuration of V5+ is [Ar]3d 04s 0, which corresponds to a closed-shell configuration. This stable configuration minimizes the energy of the system and makes V5+ less susceptible to redox reactions.
Secondly, the small size and high charge density of the V5+ ion contribute to its stability. The small size allows for strong electrostatic interactions with ligands, while the high charge density promotes the formation of strong covalent bonds.
Redox Chemistry of V5+
Despite its stability, V5+ can participate in redox reactions under certain conditions. As an oxidizing agent, V5+ can undergo reduction to lower oxidation states, such as V4+ or V3+.
- Reduction to V4+:V5+ + e –→ V4+
- Reduction to V3+:V5+ + 2e –→ V3+
Conversely, V5+ can also act as a reducing agent and undergo oxidation to higher oxidation states, such as V6+ or V7+.
- Oxidation to V6+:V5+ + e –→ V6+
- Oxidation to V7+:V5+ + 2e –→ V7+
The redox chemistry of V5+ is important in various chemical processes, including catalysis, energy storage, and environmental remediation.
Key Questions Answered
What is the purpose of an orbital diagram for V5+?
An orbital diagram for V5+ visually represents the distribution of electrons within its orbitals, providing insights into its electronic structure and bonding properties.
How many valence electrons does V5+ have?
V5+ has two valence electrons.
What is the significance of V5+ in coordination chemistry?
V5+ is a versatile ion in coordination chemistry, forming complexes with various ligands and exhibiting diverse bonding characteristics.